Is ocean iron addition part of the solution to climate change? Cliff Law, NIWA explains:
Introduction
Climate change, resulting from carbon dioxide (CO2) emissions by human activities, is one of the issues facing future global stability. Despite this awareness, current actions to reduce CO2 emissions appear to be having minimal impact on the atmospheric trend, and consequently governments and organisations are considering alternative approaches. Many of these are geoengineering techniques that require alteration of natural cycles or processes to either reduce the amount of solar radiation reaching the Earth's surface or increase the amount of CO2 removed from the atmosphere.
The oceans are an important natural sink for CO2, that have absorbed ~40% of all CO2 released since the start of the Industrial Revolution, thereby providing a buffer against climate change. About half of this CO2 uptake has been by the 'biological pump', in which photosynthesis by billions of floating phytoplankton cells in the surface waters of the ocean converts dissolved CO2 to organic matter. Upon dying the cells sink, and the carbon is exported into the deep ocean where it may be retained for hundreds of years.
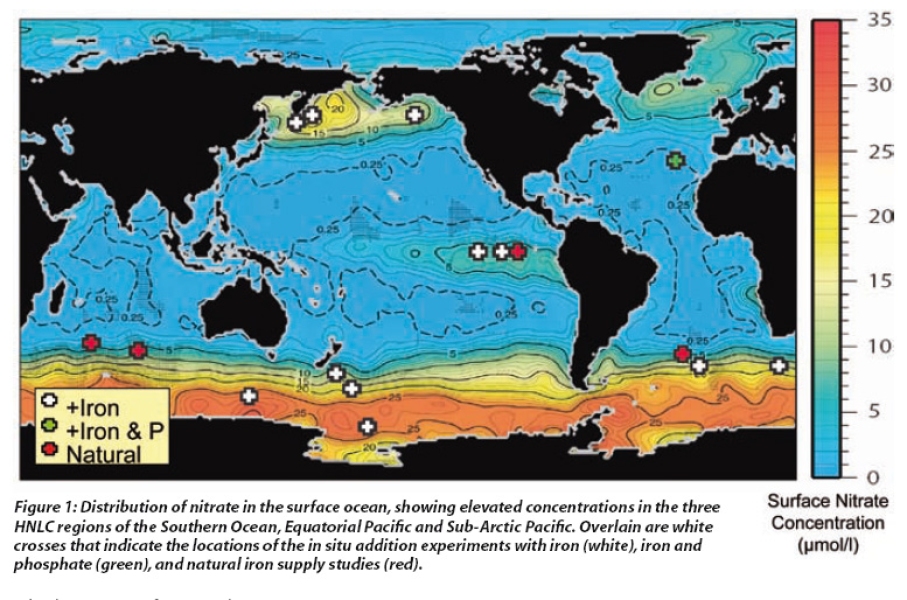
Plant growth in our gardens is determined by the availability of light and nutrients, and the same is true for phytoplankton in the surface ocean. Whereas light is generally available, a seasonal trend exists for nutrients such as nitrate and phosphate which are supplied to the surface from deep water during winter but then are rapidly removed in spring. However, in certain regions these 'macronutrients' remain perennially high in surface waters throughout spring and summer, whereas phytoplankton biomass, as indicated by the pigment chlorophyll, remains relatively low. These High-Nutrient Low-Chlorophyll (HNLC) regions include the Southern Ocean, the Equatorial Pacific and the Sub-Arctic North Pacific, and account for ~30% of the global ocean (see Figure1). This excess of macronutrients in surface waters suggests that something else is limiting phytoplankton growth in these regions.
Are parts of the open ocean anaemic?
Initially this anomaly was thought to be due to low light in these regions, but improvements in marine analytical chemistry in the 1980s led to a novel idea that lack of iron was the reason. As with many living organisms, phytoplankton also require micronutrients such as iron in trace amounts as essential components of enzyme systems. Yet, despite being one of the most abundant elements in the Earth's crust, dissolved iron is only found in low concentrations in surface waters in the open ocean. This is because the oxidised form of iron is particulate and tends to sink out. Consequently sufficient dissolved iron is only found in coastal regions and in open ocean waters that receive high dust input or upwelling. The surface waters in the HNLC regions, which are some distance from land, have perennially high macronutrients (see Figure1) and conversely extremely low dissolved iron.
The iron limitation hypothesis was championed by John Martin, a marine biogeochemist at Moss Landing Marine Laboratory, California. He supported this with evidence from bottle experiments in which the addition of dissolved iron to water from HNLC regions caused phytoplankton to grow. He also used data from ice cores, which show an inverse relationship between atmospheric CO2 and iron over the last 160,000 years. This was interpreted as indicating that during the periods in Earth's history of low iron availability, the removal of CO2 by phytoplankton photosynthesis was correspondingly low, and so atmospheric CO2 was elevated. Consequently, the iron hypothesis not only linked iron availability to phytoplankton growth, but also to oceanic carbon uptake and atmospheric CO2 concentration.
Experiments in the open ocean
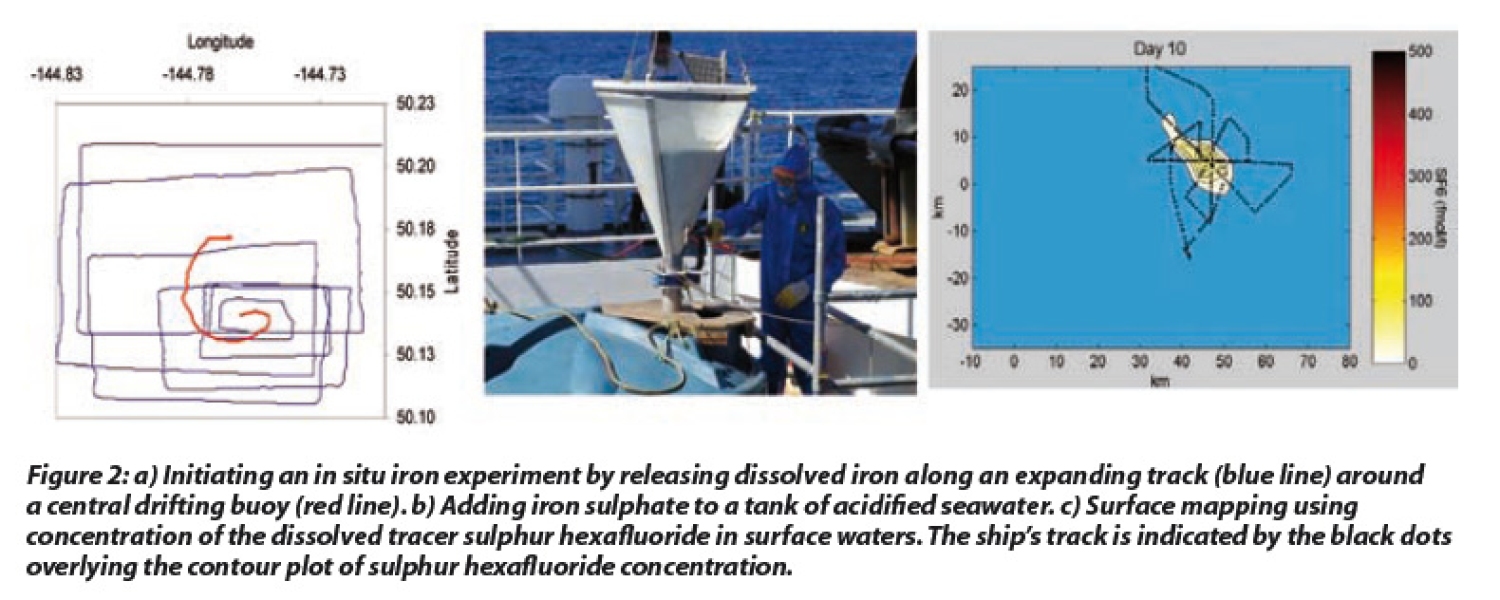
However, the evidence from the bottle incubations was not conclusive, and so the next step was to directly release dissolved iron into the surface waters of HNLC regions to determine whether phytoplankton growth would be stimulated in situ. Initiating an in situ experiment is not as simple as it may initially seem, due to the dynamic nature of the ocean. For example, it is easier to test whether fertiliser causes a crop to grow on an area of agricultural land, than in a patch of surface ocean water that is moving, spreading and mixing with surrounding water. To account for this during the in situ iron experiments, the dissolved iron is released along an expanding track around a central drifting buoy, resulting in a coherent patch of fertilised water of around 50-100 km2 (see Figure 2).
Whereas the approach in these experiments has often been incorrectly described as 'dumping iron filings', most experiments have instead used single or repeat additions of 0.5-3 tonnes of dissolved iron sulphate. Although this may sound a large volume, when distributed over 50 km2 it only raises the background dissolved iron by 20-40 fold. Although usually acidified to pH 2 to maintain it in a soluble form, the iron is still rapidly oxidised and lost from surface waters, so in order to follow the fertilised patch of water a tracer is also added. Sulphur hexafluoride, a dissolved gas that is detectable at incredibly low concentrations, is used as it permits tracking even when a patch covers an area exceeding 1,000 km2. During a typical experiment, the patch position and boundaries are determined by mapping sulphur hexafluoride in surface water (see Figure 2), and the water at the centre of the patch (the 'IN station') is then compared with water outside the patch (the 'OUT station') to determine if the added iron has had an effect.
Iron grows phytoplankton
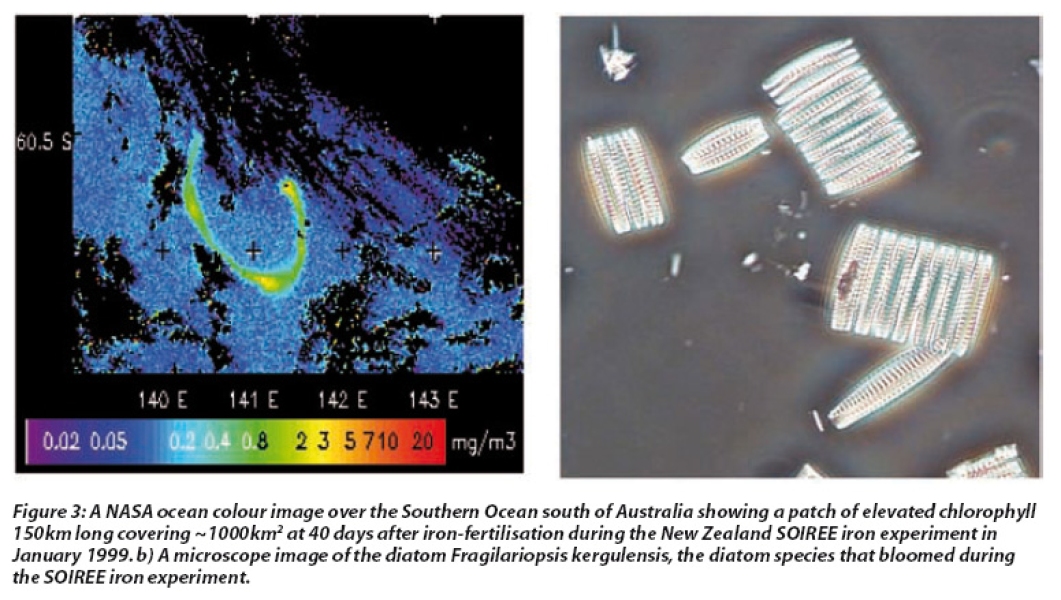
The first in situ iron addition experiments took place in the Equatorial Pacific during October 1993, and have subsequently been followed by twelve further experiments in this and other regions (see Figure 1). Most experiments have shown consistent increases in phytoplankton biomass, albeit of different magnitudes, with chlorophyll increasing 2-14 fold at the patch centre. The resulting patches, which often exceed 1,000 km2 in surface area, have been observed in satellite images of ocean colour (see Figure 3a).
In most experiments phytoplankton cell size has generally increased due to a shift in the type of phytoplankton, with the smaller phytoplankton that dominate in HNLC regions giving way to larger phytoplankton. The latter are often diatoms, a group of phytoplankton with silicate shells that are important in the transport of organic carbon to the deep ocean (see Figure 3).
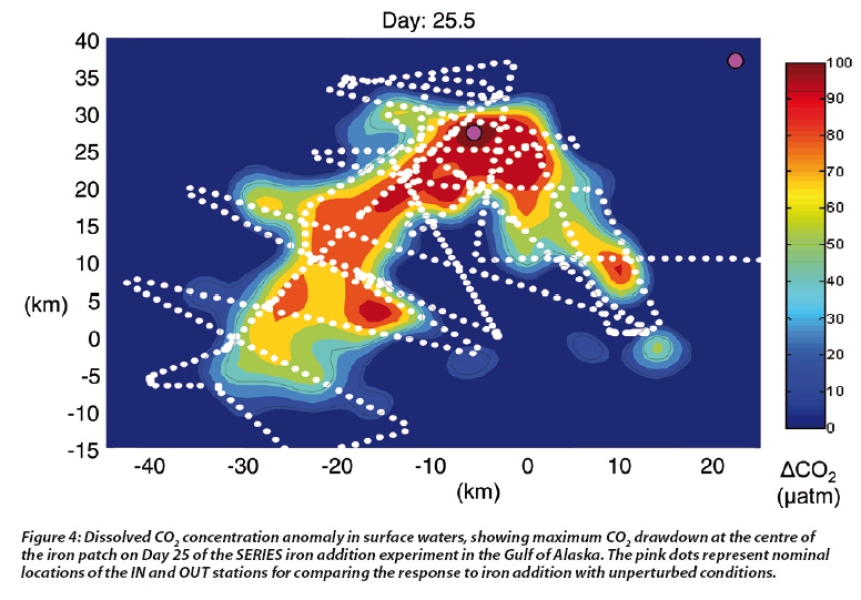
This increase in phytoplankton growth in the iron fertilised patch stimulates drawdown of dissolved CO2 (see Figure 4), which in some experiments has decreased at the patch centre by 40%. This coincides with increased uptake of macronutrients, with nitrate, phosphate and silicate declining at the centre of the iron patch, often to levels that begin to limit phytoplankton growth. The phytoplankton response has varied in different in situ iron experiments due to differences in light availability, patch mixing and dilution, and grazing by zooplankton. Nevertheless, these in situ experiments have confirmed the first part of the iron hypothesis: that iron availability limits phytoplankton in HNLC waters.
Photosynthesis does not equal carbon export
However, few of the in situ experiments have measured the export of the carbon fixed by the phytoplankton into the sub-surface ocean. This reflects that the motivation behind the in situ iron experiments was to understand what controls phytoplankton growth and how marine ecosystems respond to change in nutrient supply, rather than whether iron addition can increase ocean carbon uptake.
The in situ experiments that have measured carbon export have identified that only 2-20% of the carbon fixed by phytoplankton in the iron fertilised patch is subsequently transferred to sub-surface waters. This is consistent due to non-fertilisation studies, and is due to the majority of sinking phytoplankton being broken down by grazing and bacterial activity and converted back into inorganic nutrients and CO2 before reaching deep water. Consequently although iron addition stimulates phytoplankton growth, it remains unclear whether it significantly increases the transfer of carbon from atmosphere to the deep ocean.
Geoengineering interest
Nevertheless, from the outset there has been interest in using this approach at larger scales to increase phytoplankton growth and enhance the ocean carbon sink. This can be partly attributed to John Martins' attention grabbing quote: "Give me half a tanker of iron, and I'll give you the next ice age". The subsequent in situ iron experiments further reinforced the potential, particularly with the unambiguous visual demonstration of iron-induced phytoplankton growth in satellite ocean colour images (see Figure 3a).
Furthermore, initial laboratory studies indicated that as phytoplankton require iron in very low quantities, iron addition represented a potentially cheap approach to removing carbon. With the development of an active carbon trading market over the last 5-10 years, commercial organisations are now promoting large-scale ocean iron fertilisation, with the claim that it offers the greatest potential for carbon sequestration of all available techniques.
Is it effective?
Evidence indicates that large-scale iron fertilisation may not be such an effective approach to lowering atmospheric CO2. One of the main attractants for the commercial sector is the apparent large 'bang-for-buck' of iron addition. The early laboratory studies of phytoplankton cultures suggested a ratio of carbon fixed to iron added on the order of 110,000, with an estimated cost of a few dollars per tonne of carbon fixed. However, subsequent in situ experiments have shown a lower ratio of carbon exported to iron added, of 1,000-30,000, and so a higher cost per tonne of carbon of >$300 making it considerably less attractive.
The lower carbon:iron ratio estimated from the in situ iron experiments results in part from the rapid loss of added iron, which could possibly be minimised in future releases by addition of iron in an organic form. However, estimates of carbon uptake by large-scale iron fertilisation using ocean models have also decreased, as a result of improvements in both the models and baseline information on processes and fluxes. Current estimates now suggest that large-scale (global ocean) long-term (100-year) fertilisation with iron would draw down atmospheric CO2 by 33ppm, which is <9% of current atmospheric CO2 levels. So although ocean iron addition isn't the answer to climate change, could it be part of the solution? It has been suggested that iron fertilisation could be just one of a portfolio of technological approaches to lowering atmospheric CO2. However, recent re-analysis of ice core data suggests that the rate of CO2 drawdown resulting from iron addition may be too slow to be of use.
Verification is difficult
There are also other criteria that must be considered in addition to effectiveness. The carbon regulatory market requires further standards for carbon sinks, which include verification of carbon storage, and permanence, by which carbon must be retained for 100 years or greater. Again, using the previous analogy of crops on agricultural land, it is easier to follow the transfer and storage of carbon in a terrestrial system than in the more dynamic ocean. Not only will the carbon fixed by phytoplankton sink 1000s of metres through the water column, but it is also transported laterally over 1000s of kilometres by ocean currents.
Determining the permanence of carbon sequestration in the ocean is therefore highly challenging, expensive and currently not considered viable with existing technology. Yet this is essential, particularly as the 100-year time horizon, which is generally considered to be depths greater than 500 m, is only reached in certain regions, with sub-surface water returning to the surface within decades in other regions.
Potential side effects
A further criterion of the carbon trading market is no side effects, and yet there are a number of potential effects of iron fertilisation both locally and at distance from the fertilisation site. For example, the in situ iron experiments have demonstrated that iron addition causes an increase in large phytoplankton groups, and so a change in biodiversity that may impact further up the food chain. At present this is viewed as positive, as increased phytoplankton biomass may lead to an increase in consumers and fish stocks. However, there is concern that fertilisation may also stimulate the development of toxic algal blooms. Although this has not been observed in any in situ iron experiments, recent evidence indicates that the diatom species that respond to iron addition also increase their production of neurotoxin.
Concern has also been raised regarding the 'far-field' effects of ocean fertilisation. As phytoplankton decompose and dissolved oxygen is consumed, this may cause expansion of low oxygen zones in the mid-water column. The spatial extent of 'dead zones' of very low oxygen content has increased particularly in upwelling regions, and this could be further exacerbated by large-scale iron fertilisation.
Associated with oxygen removal is the increased production of other gases during microbial decomposition. This includes nitrous oxide, another greenhouse gas that has a global warming potential 300 times that of CO2 on a molecular basis. An increase in nitrous oxide production in the mid-water column and subsequent ventilation to the atmosphere may negate any benefits gained from the CO2 drawdown stimulated by iron-induced phytoplankton growth. To date there has been no evidence of this from the in situ iron experiments, although ocean models suggest that this offsetting effect of nitrous oxide production may be significant dependent upon the location of iron addition.
Conversely increased production of sulphurcontaining gases has been observed in some in situ iron experiments, which on larger scales could increase cloud formation and potentially enhance cooling of the planet. A further concern is that of 'nutrient-robbing' by which enhanced macronutrient uptake by phytoplankton due to iron addition in one region may deprive another region downstream of nutrients and thereby reduce productivity.
Legislation
These potential side effects are just one of the reasons why large-scale iron fertilisation remains a contentious issue. Other uncertainties, such as the sourcing and transport of the huge volumes of iron required for large-scale addition over periods of 10-100 years, and the use of large areas of the ocean commons for which no relevant regulations or jurisdiction exist also represent major sticking points. The current interest in large-scale iron fertilisation by commercial organisations, combined with these uncertainties, has led to statements of concern by leading environmental organisations. This in turn has stimulated recent activity in the development of international legislation.
A statement issued by the International Maritime Organisation which administers the London Convention, the main legal instrument for controlling marine pollution, has decreed that ocean fertilisation activities other than legitimate scientific research are prohibited. An assessment framework for establishing the legitimacy of future scientific in situ iron experiments is currently under development. Currently there are no firm plans to undertake large-scale iron addition experiments; however, the continuing increase in atmospheric CO2 and development of the global carbon trading market will undoubtedly see further activity and research on ocean iron fertilisation at larger scales.
(This article first appeared in NZ Teachers magazine in 2010)
Further reading
- Bertram C. (2010) Ocean iron fertilization in the context of the Kyoto protocol and the post-Kyoto process. Energy Policy 38, 1130-9.
- Boyd P.W. et al (2007) Mesoscale iron enrichment experiments 1993-2005: synthesis and future directions. Science 315, 5812, 612-7.
- Buesseler K.O. et al (2008) Ocean iron fertilization – moving forward in a sea of uncertainty. Science 319, 162.
- Royal Society (2009) Geoengineering the climate: science, governance and uncertainty. RS Policy Document 10/09.
- Strong A.L., Cullen J.J., & Chisholm S.W., (2009) Ocean fertilization. Science, policy and commerce. Oceanography 22, 236-61.
- 12 Papers in the Theme Section on Implications of large-scale iron fertilization of the oceans, Marine Ecology Progress Series (2008); vol 364, 213-309.